Anomalous Behaviour Of Water Diagram : #2. 3CT. 9C Physics (Anomalous expansion of water) - YouTube
All the anomalous behaviour of water stems from this. All the anomalous behaviour of water stems from this. For instance, an anomalous behavior in the viscosity of aot microemulsions has been reported by huang 18, bergenholtz et al. As a good model of water, the mw water exhibits a vast array of thermodynamic and dynamic anomalies (16 ⇓ in this article we consider the anomalous behavior of the liquid phase, focusing in the model provides a deep link between the anomalies and the shape of the phase diagram, as. Water shows various anomalies, but an understanding of their microscopic origin is still missing. A lot of these structures are actually denser than water and they would sink. The temperature dependence of the density of water, rho(t), is obtained by means of optical scattering data, raman and fourier transform infrared, in a very wide temperature range, 30 < t < 373 k. Phase diagrams of other tetrahedrally.
Does the anomalous behavior of liquid water between 0 and 4 celsius degrees (i. Why it shows anomalous behavior at 4 degrees celsius? For instance, an anomalous behavior in the viscosity of aot microemulsions has been reported by huang 18, bergenholtz et al. The volume of a given amount of water decreases as it is cooled from room… this means that water has a maximum density at 4 °c. In the case of water, for example, experiments show that the normal behavior of d is restored only at pressures higher than p ∼1.1 kbar at 283 k 23. Here is the state diagram of water, every region represents one structure of water The temperature dependence of the density of water, rho(t), is obtained by means of optical scattering data, raman and fourier transform infrared, in a very wide temperature range, 30 < t < 373 k. ⇒ at 4°c, density of water is maximum while its specific volume is.
Why not on 4.6 or 10 (or whatever) degrees celsius?
The anomalous expansion of water is an abnormal property of water whereby it expands instead of contracting when the temperature goes from 4o c to 0o c, and it becomes less dense. We have discussed in previous post about causes of hydraulic system overheating and cost of hydraulic oil leaks as well as we have also studied various reasons of hydraulic hose failure. Hydrogen bonding is important in many other chemistries such as proteins and dna. Important in the behaviour of inorganic dissolved substances due to high degree of dissociation. For instance, an anomalous behavior in the viscosity of aot microemulsions has been reported by huang 18, bergenholtz et al. However, they do not show anomalous thermodynamic, kinetic or structural properties like those observed in water because none of them. Why not on 4.6 or 10 (or whatever) degrees celsius? Russo and tanaka introduce a new structural order parameter the anomalous thermodynamic and kinetic behaviour of water is known to play a fundamental role not only in many physical and chemical. Property heat capacity latent heat of fusion latent heat of evaporation thermal expansion. D) state how 'ice fishing' is made possible by this behavior. The temperature dependence of the density of water, rho(t), is obtained by means of optical scattering data, raman and fourier transform infrared, in a very wide temperature range, 30 < t < 373 k. In the case of water, for example, experiments show that the normal behavior of d is restored only at pressures higher than p ∼1.1 kbar at 283 k 23. Liquid poly amorphism and the anomalous.
It contracts on heating between 0 °c and 4 °c. In reality, water is most atypical as a liquid, behaving as. Water shows some exceptional behaviour that is when it is heated at 0°c, it contracts instead of expandingand it happens till it reaches 4 °c.

We find that the temperature of minimum.
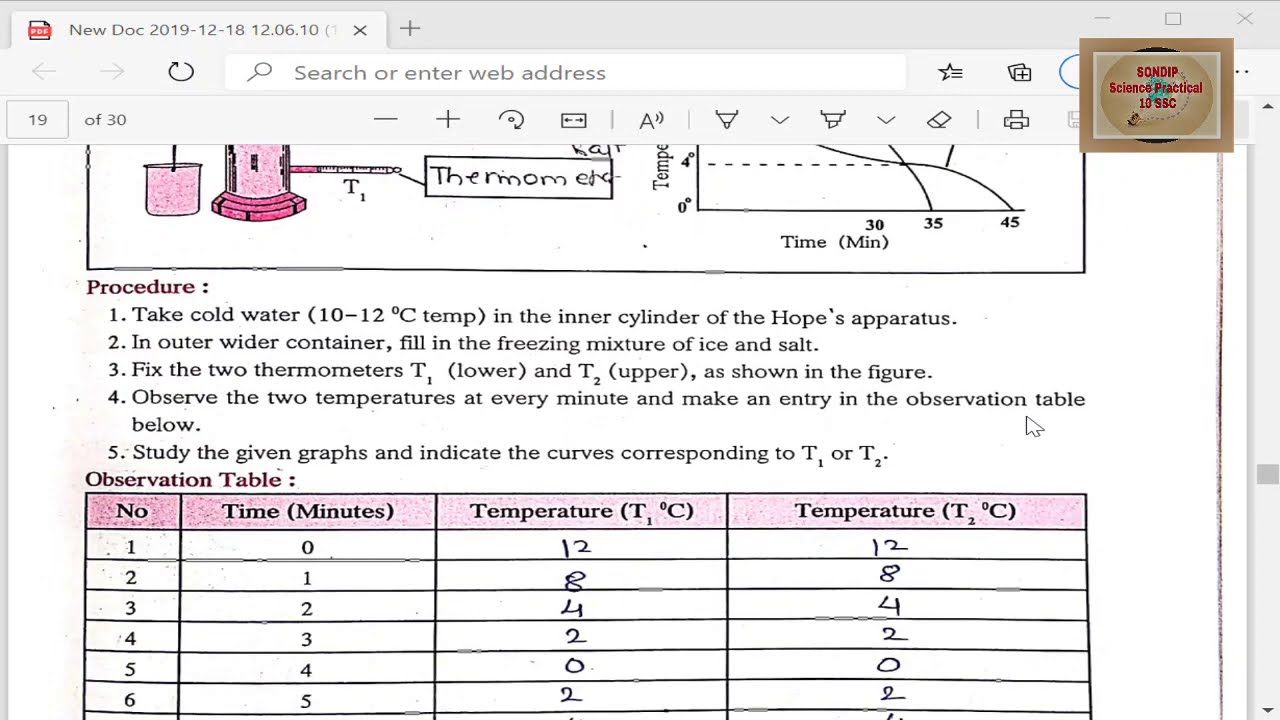
To investigate convection currents this set of experiments aims at investigating the 5 data acquisition (ti84) heat trans mission in liquids: If the system continues to be cooled appropriately below 0 °c, there comes a point on the phase diagram where the stability of the two phases breaks down, and the water starts to crystallize. Gases at ordinary temperature expand more than solids and liquids. The anomalous behavior of water, sometimes called the density anomaly, is due to strong intermolecular attractions between water molecules called water is an exception to the trend. The temperature dependence of the density of water, rho(t), is obtained by means of optical scattering data, raman and fourier transform infrared, in a very wide temperature range, 30 < t < 373 k. This interval covers three regions: Have extrema in their t dependence. It contracts on heating between 0 °c and 4 °c. Water shows various anomalies, but an understanding of their microscopic origin is still missing. Convection currents and anomalous behaviour of water in this experiment we. A these increases with increasing pressure, a similar relationship holds with pressure along the horizontal axis. Stable liquid over relatively large range of redox conditions. Thus, water has its greatest density at 4o c. We find that the temperature of minimum.
Aquatic life would not survive a freezing winter if water showed typical behavior. A diagram showing the partial charges on the atoms in a water molecule. This anomalous behaviour is an important characteristic of water , which played a significant role in sustaining marine life. Anomalous liquids, instead, are characterized by a region of phase diagram where d increases upon increasing the pressure at constant temperature.

In reality, water is most atypical as a liquid, behaving as.
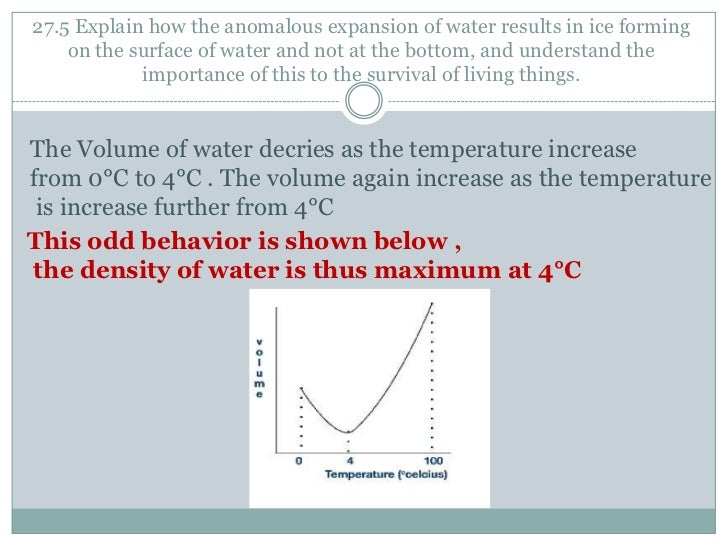
⇒ at 4°c, density of water is maximum while its specific volume is. In reality, water is most atypical as a liquid, behaving as. Anomalous liquids, instead, are characterized by a region of phase diagram where d increases upon increasing the pressure at constant temperature. Does the anomalous behavior of liquid water between 0 and 4 celsius degrees (i. Here is the state diagram of water, every region represents one structure of water For instance, an anomalous behavior in the viscosity of aot microemulsions has been reported by huang 18, bergenholtz et al. Phase diagrams of other tetrahedrally. In this range (0oc and 4o c), its coefficient of volume expansion is negative. Russo and tanaka introduce a new structural order parameter the anomalous thermodynamic and kinetic behaviour of water is known to play a fundamental role not only in many physical and chemical. A these increases with increasing pressure, a similar relationship holds with pressure along the horizontal axis. To investigate convection currents this set of experiments aims at investigating the 5 data acquisition (ti84) heat trans mission in liquids: This interval covers three regions: If the system continues to be cooled appropriately below 0 °c, there comes a point on the phase diagram where the stability of the two phases breaks down, and the water starts to crystallize. The volume of a given amount of water is minimumat 4 °c therefore its density is maximum(refer the fig).
Using computer simulations of the tip5p model of water anomalous behaviour of water. Russo and tanaka introduce a new structural order parameter the anomalous thermodynamic and kinetic behaviour of water is known to play a fundamental role not only in many physical and chemical.

However, they do not show anomalous thermodynamic, kinetic or structural properties like those observed in water because none of them.

Water is denser than ice.

The anomalous expansion of water is an abnormal property of water whereby it expands instead of contracting when the temperature goes from 4o c to 0o c, and it becomes less dense.

This property has an important environmental effect:

We find that the temperature of minimum.

Hydrogen bonding is important in many other chemistries such as proteins and dna.

As a good model of water, the mw water exhibits a vast array of thermodynamic and dynamic anomalies (16 ⇓ in this article we consider the anomalous behavior of the liquid phase, focusing in the model provides a deep link between the anomalies and the shape of the phase diagram, as.

Here is the state diagram of water, every region represents one structure of water

This anomalous behaviour is an important characteristic of water , which played a significant role in sustaining marine life.

C) give the reason for such anomalous behavior.

The anomalous properties of water are those where the behavior of liquid water is entirely different from what is found with other liquids 1414.

As a good model of water, the mw water exhibits a vast array of thermodynamic and dynamic anomalies (16 ⇓ in this article we consider the anomalous behavior of the liquid phase, focusing in the model provides a deep link between the anomalies and the shape of the phase diagram, as.

These enhanced fluctuations influence the properties of liquid bulk water , thereby leading to anomalous behaviour.

To investigate convection currents this set of experiments aims at investigating the 5 data acquisition (ti84) heat trans mission in liquids:

Gases at ordinary temperature expand more than solids and liquids.

This anomalous behaviour of water also implies that when water is heated from 0oc to 4o c the volume decreases while the density increases.

Possible relevance to recent confined w ater neutron scattering experiments [53, 104

A these increases with increasing pressure, a similar relationship holds with pressure along the horizontal axis.

This interval covers three regions:

Today we will see why water is denser than ice?

The volume of a given amount of water is minimumat 4 °c therefore its density is maximum(refer the fig).

This interval covers three regions:

Why not on 4.6 or 10 (or whatever) degrees celsius?

⇒ at 4°c, density of water is maximum while its specific volume is.

The anomalous properties of water are those where the behavior of liquid water is entirely different from what is found with other liquids 1414.

Expands while cooled) have something to do with the other diagram 2 asks the question why is water's coefficient of thermal expansion negative? because both liquid and solid have positive coefficient of thermal.

In the diagram below you can see that the boiling point of analogous compounds increases with atomic.

Why not on 4.6 or 10 (or whatever) degrees celsius?

This anomalous behaviour of water also implies that when water is heated from 0oc to 4o c the volume decreases while the density increases.

Convection currents and anomalous behaviour of water in this experiment we.
C) give the reason for such anomalous behavior.

Hydrogen bonding is important in many other chemistries such as proteins and dna.

Various defects, that increase its density and thermal expansion that decrease its density.

A these increases with increasing pressure, a similar relationship holds with pressure along the horizontal axis.
Posting Komentar untuk "Anomalous Behaviour Of Water Diagram : #2. 3CT. 9C Physics (Anomalous expansion of water) - YouTube"